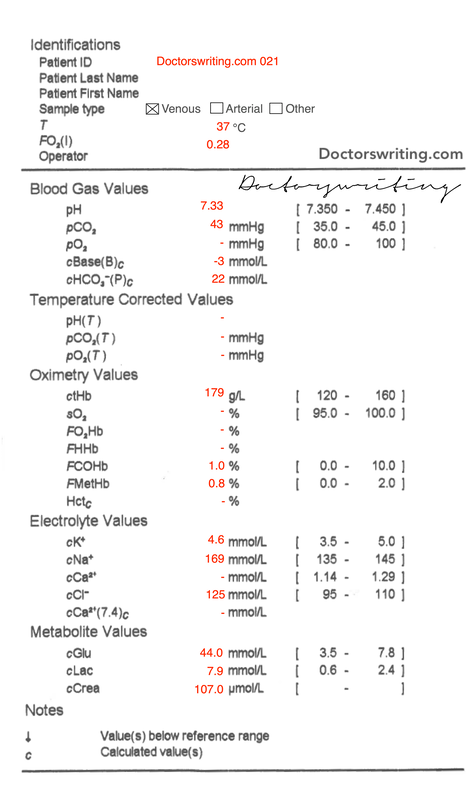
Cause of acidaemia?
- CO2 = 43 (within normal range)
- HCO3 = 22 (within normal range)
...hang on...so what is the cause?
This is a good blood gas to highlight that the model we use to interpret blood gasses has its limitations.
It is based on the Henderson-Hasselback Equation (below) with regards to the bicarbonate buffer system and assumes that carbonic acid and CO2 equilibrate without interaction with other ions in the plasma.
The Strong Ion Theory is based around the fact there are some ions in the body that affect the equilibrium of the Henderson-Hasselback model. The most important being chloride (Cl-) the ion of the chlorine element.
Chlorine has the chemical property that it is missing 1 negatively charged electron from its outer shell. It is much more stable when is gains that extra electron making a Cl- ion. This effect is so strong it will actively “strip” electrons from hydrogen atoms forming H+ ions. H+ is of course acid. This moves the equilibrium of the above Henderson-Hasselback to a more acidic level than one would expect for the values of CO2 and HCO3.
So in our patient his Cl- is 125.
Although his HCO3 and CO2 should not cause his acidosis, his high chloride has done so.
He has a hyperchloraemic metabolic acidosis.
For this reason our commonly used calculations for compensation are not usable in this case as our “standard” acid-base interpretation model has reached the limit of its usability.
*Note the use of the words “more complicated” not “better”. A scientific model is a mathematical and/or conceptual representation of a system of ideas, events or processes. The “best” model is the one that gives usable results in the simplest way possible.
- Actual Na = mNa + (Glc - 5)/3 = 169 + (44 - 5)/3 = 182
- ∴ Severe hypERnatraemia
K (for pH)
- Expected K = 5(7.4 - pH) + 5 = 5(7.4 - 7.33) + 5 = 5.35
- Actual K = 4.6 ∴ 𝛥K = -0/75
- ∴ Normal plasma hypERkalaemia (mild total body hypOkalaemia)
Ca (for alb)
- No albumin level to correct to
Cl (no correction req)
- Cl = 125 ∴ moderate hypERchloraemia
Glc very high
Hb high - apparent polycythemia
Crea mildly elevated
COHb normal
MetHb normal
Bedside ketones help seal the deal (normal of course)
As a recap, the 'diagnostic' criteria for HHS are:
- Serum glucose > 33mmol/L
- No ketoacidosis (and normally a normal pH)
- Profound dehydration (elevated urea:creatinine ratio)
- Serum osmolarity > 320mosmol/L
So, does the VBG support this?
Absolutely!
- The Glucose is 44 so greater than the diagnostic criteria
- The calculated osmolarity is 2 x Na + Urea + Glucose. Even without knowing the urea the total is 382. Well above the diagnostic criteria!
- Hyperchloraemic metabolic acidosis
- Polycythaemia
- Lactate 7.9 with a normal pH (and normal pCO2)
Hyperchloraemic metabolic acidosis
The VBG shows a hyperchloraemic metabolic acidosis. The hyperchloraemia may be due to profound dehydration or prior administration of IVF.
Polycythaemia
The polycythaemia is likely another indicator of dehydration, but could be due to a secondary condition such as smoking related lung disease or polycythaemia rubra vera.
Why is the lactate 7.9?
This may be another sign of dehydration, shock and hypoperfusion. However, in a HHS patient one must consider metformin toxicity as this is a likely medication a type 2 diabetic would be taking.
Why is this patient not more acidotic?
If we look at the VBG as a whole it is surprising that the pH is 7.33. Maybe there's a concominant metabolic alkalosis? We can work that out through the delta ratio:
- DR = [22-12]/[24-22] = 5
- DR > 2 = HAGMA & Met Alkalosis! But wait...
As we stated in part one, due to the raised chloride, our regular model of gas interpretation may not be ideal in these circumstances.
To double check, we can use a physiochemical approach using the strong ion calculations; Strong Ion Difference (SID) and Strong Ion Gap (SIG):
- SID = Na - Cl
- SID < 38 = Metabolic Acidosis
- SID > 38 = Metabolic Alkalosis
- SIG = (Base deficit) + (SID - 38) + 2.5 (4.2 - Alb (g/dL)) - Lactate
- Think of SIG like your anion gap...but with more variables (so less error)
- SIG > 2 = metabolic acidosis (mostly uraemia, DKA, AKA< toxins, lactate)
- SIG < 2 = Hypercalcemia, Hypermagnesemia, Hyperkalemia, Immunoglobulins, Bromide, Nitrates, Lithium Overdose
For this patient (assuming normal albumin = 3.5):
- SID = 44 = metabolic alkalosis
- SIG = 3 + (44-38) + 2.5(4.2-3.5) - 7.9 = 2.85 = metabolic acidosis
So why is there a metabolic acidosis (SIG) and metabolic alkalosis (SID)?
- SIG metabolic acidosis due to a raised lactate
- SID metabolic alkalosis possibly caused by vomiting in a patient this unwell with HHS
There are 2 approaches to analysing blood gases. The pragmatic method, what you already know, and the strong ion calculations...a more pure assessment that requires additional cognitive load for little additional clinical benefit. Perhaps the main utility in the clinical setting is when the Cl- is massively raised and/or the blood gas doesn't make sense and you want to check your work!
Specific aspects of HHS Management
- Correct severe dehydration
- Correct electrolytes (and when you replace K, replace Mg too)
- Give insulin
- Seek and Treat the underlying cause
- VTE prophylaxis
1. Dehydration (First 24 hours)
- Calculate H2O deficit = 0.6 x usual weight x (1 – 140/cNa)
- Replace half the H2O deficit over 24 hours using ½ normal saline
- Do not reduce Na by more than 10mmol/day
- Hypernatraemia correct with dehydration management
- Plasma K will likely drop...unless anuric
- When replacing K, replace Mg (those distal tubular ROMK channels are needy...they wont reabsorb K unless they get some Mg from plasma!)
- Insulin given at 0.05 U/kg/hr, aiming for a gradual drop (max 3 mmol/L/hr)
- Comprehensive workup!
- Risk is high - about 1.2% with a HR 3.0 compared to non-hyperosmolar hyperglycaemia (including DKA)

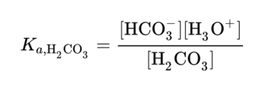

 RSS Feed
RSS Feed
